
Characteristics of brown zone formed by weathering around fracture in the Hiroshima granite and estimation of iron source for iron oxide precipitation
ABSTRACT
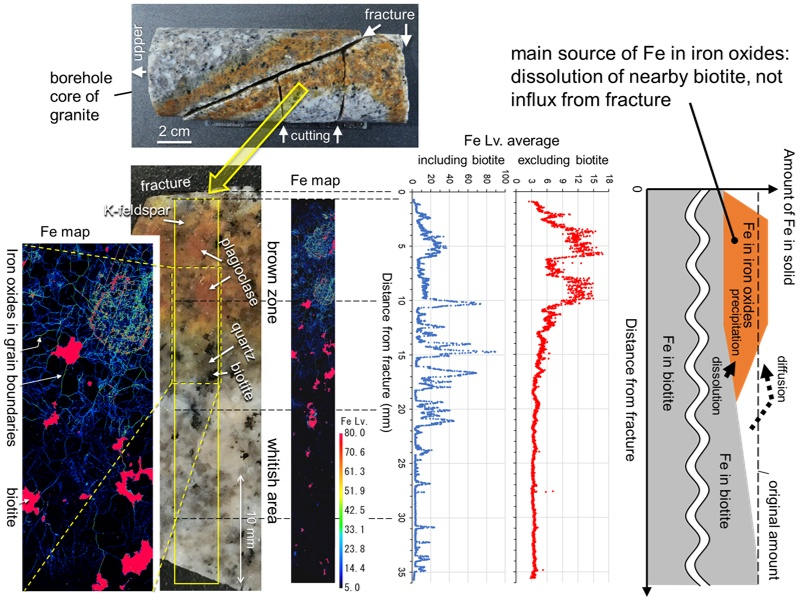
Brownish zones enriched in iron (oxyhydr)oxides often occur around fractures during granite weathering. The elemental distribution, color, iron oxide concentration, mineral assemblage, porosity, and iron diffusivity in the brownish zone around a fracture in the Hiroshima granite were investigated. From the fracture to the interior of the rock matrix, the iron concentration first increased with distance from the fracture, reaching a maximum at 4–10 mm, decreasing at 10–21 mm, and remaining constant in the whitish areas at greater distances. Iron oxides, thought to be mainly goethite and ferrihydrite, were distributed at the grain boundaries and concentrated in the altered portions of plagioclase. The selective iron dissolution method revealed that 0.32 wt% Fe is present as iron oxides in the brown zone. Numerical calculations of the reaction and transport of Fe near the fracture showed the following: under conditions where Fe does not precipitate, the dissolution of biotite takes less time to supply Fe for the formation of iron oxides than the diffusion of Fe from groundwater in the fracture; under conditions where Fe precipitates, the Fe that diffuses from the fracture almost completely precipitates in the vicinity of the fracture and is unlikely to be a major source of iron oxide in the brown zone. A large proportion of the brown zone Fe appears to have been derived from the dissolution of nearby biotite.
KEYWORDS
Keywords: iron oxide, granite, weathering, diffusion, dissolution- Published : 2024
- Released on J-STAGE : 2024/10/16
- Received : 2023/12/10
- Accepted : 2024/08/13
- DOI : https://doi.org/10.2343/geochemj.GJ24017
- J-STAGE URL : https://www.jstage.jst.go.jp/article/geochemj/58/5/58_GJ24017/_article/-char/en
- J-Online ISSN: 1880-5973
- Print ISSN : 0016-7002
- ISSN-L : 0016-7002
All Issues
- Vol.59, 2025
- Vol.58, 2024
- Vol.57, 2023
- Vol.56, 2022
- Vol.55, 2021
- Vol.54, 2020
- Vol.53, 2019
- Vol.52, 2018
- Vol.51, 2017
- Vol.50, 2016
- Vol.49, 2015
- Vol.48, 2014
- Vol.47, 2013
- Vol.46, 2012
- Vol.45, 2011
- Vol.44, 2010
- Vol.43, 2009
- Vol.42, 2008
- Vol.41, 2007
- Vol.40, 2006
- Vol.39, 2005
- Vol.38, 2004
- Vol.37, 2003
- Vol.36, 2002
- Vol.35, 2001
- Vol.34, 2000
- Vol.33, 1999
- Vol.32, 1998
- Vol.31, 1997
- Vol.30, 1996
- Vol.29, 1995
- Vol.28, 1994
- Vol.27, 1993
- Vol.26, 1992
- Vol.25, 1991
- Vol.24, 1990
- Vol.23, 1989
- Vol.22, 1988
- Vol.21, 1987
- Vol.20, 1986
- Vol.19, 1985-1986
- Vol.18, 1984
- Vol.17, 1983
- Vol.16, 1982
- Vol.15, 1981
- Vol.14, 1980
- Vol.13, 1979
- Vol.12, 1978
- Vol.11, 1977
- Vol.10, 1976
- Vol.9, 1975
- Vol.8, 1974
- Vol.7, 1973
- Vol.6, 1972-1973
- Vol.5, 1971
- Vol.4, 1970-1971
- Vol.3, 1969-1970
- Vol.2, 1968
- Vol.1, 1966-1967




