
Nuclear volume and mass dependent fractionation of cerium isotopes
ABSTRACT
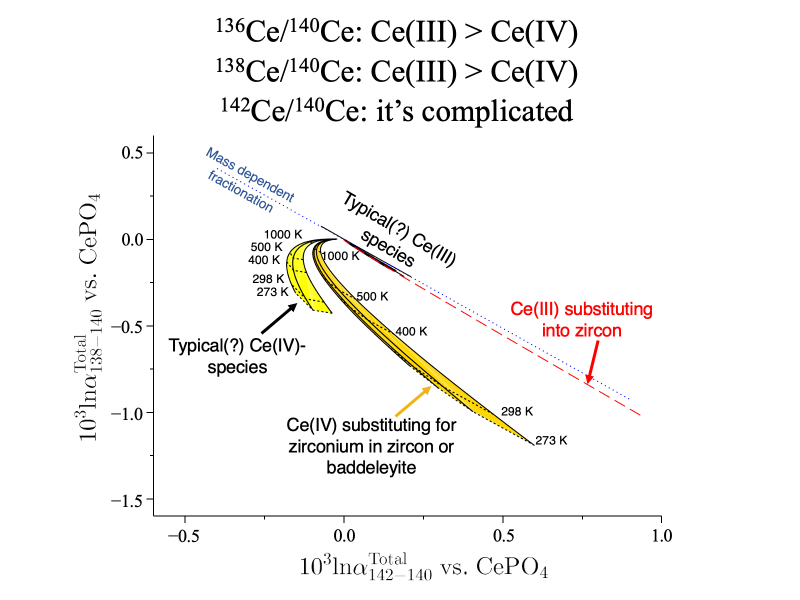
Equilibrium cerium isotope fractionations in cerium-bearing minerals and aqueous species are estimated using electronic structure calculations that include both nuclear volume and mass dependent effects. As with europium and uranium, the nuclear volume effect in redox reactions goes in the opposite direction from equilibrium mass-dependent fractionation for 142Ce/140Ce because of the larger nuclear charge radius of the 142Ce nucleus. Mass-dependent effects dominate 136Ce/140Ce and 138Ce/140Ce fractionations because 136Ce, 138Ce, and 140Ce share very similar nuclear charge radii. 142Ce/140Ce is predicted to be lower in most Ce(IV)-bearing species than in coexisting Ce(III)-bearing species, particularly at high temperatures. However, species with Ce:Zr substitution, such as zirconium-rich compositions along the stetindite-zircon (CeSiO4-ZrSiO4) solid solution series and a model Ce-subsituted baddeleyite (CeZr3O8), may show higher 142Ce/140Ce at ambient to low-T metamorphic temperatures because of higher effective force constants acting on the smaller, more snug substitution sites. Ce(III)P(V) charge-coupled substitution into zircon is likewise associated with high 142Ce/140Ce relative to other Ce(III) species. 136Ce/140Ce and 138Ce/140Ce fractionations will tend to favor more massive isotopes in Ce(IV)-bearing species, by 0.1–1.0‰ at 25°C and 0.01–0.1‰ at 727°C depending on the species present. The models predict ~0.3‰ higher 142Ce/140Ce in Ce(III)-bearing solution than coexisting Ce(IV)-solids at ambient temperatures, roughly agreeing with measurements. Zircon in equilibrium with typical silicate melts is predicted to be slightly enriched in 140Ce relative to 142Ce, 138Ce, and 136Ce. Supplementary calculations based on 141Pr-Mössbauer spectroscopy literature suggest somewhat (~1/3) smaller nuclear volume fractionation effects than the electronic structure models.
KEYWORDS
Keywords: cerium isotopes, nuclear field shift, rare earth element isotope fractionation, cerium anomaly, zircon- Published : 2024
- Released on J-STAGE : 2024/11/19
- Received : 2024/06/05
- Accepted : 2024/09/09
- DOI : https://doi.org/10.2343/geochemj.GJ24019
- J-STAGE URL : https://www.jstage.jst.go.jp/article/geochemj/58/6/58_GJ24019/_article/-char/en
- J-Online ISSN: 1880-5973
- Print ISSN : 0016-7002
- ISSN-L : 0016-7002
All Issues
- Vol.59, 2025
- Vol.58, 2024
- Vol.57, 2023
- Vol.56, 2022
- Vol.55, 2021
- Vol.54, 2020
- Vol.53, 2019
- Vol.52, 2018
- Vol.51, 2017
- Vol.50, 2016
- Vol.49, 2015
- Vol.48, 2014
- Vol.47, 2013
- Vol.46, 2012
- Vol.45, 2011
- Vol.44, 2010
- Vol.43, 2009
- Vol.42, 2008
- Vol.41, 2007
- Vol.40, 2006
- Vol.39, 2005
- Vol.38, 2004
- Vol.37, 2003
- Vol.36, 2002
- Vol.35, 2001
- Vol.34, 2000
- Vol.33, 1999
- Vol.32, 1998
- Vol.31, 1997
- Vol.30, 1996
- Vol.29, 1995
- Vol.28, 1994
- Vol.27, 1993
- Vol.26, 1992
- Vol.25, 1991
- Vol.24, 1990
- Vol.23, 1989
- Vol.22, 1988
- Vol.21, 1987
- Vol.20, 1986
- Vol.19, 1985-1986
- Vol.18, 1984
- Vol.17, 1983
- Vol.16, 1982
- Vol.15, 1981
- Vol.14, 1980
- Vol.13, 1979
- Vol.12, 1978
- Vol.11, 1977
- Vol.10, 1976
- Vol.9, 1975
- Vol.8, 1974
- Vol.7, 1973
- Vol.6, 1972-1973
- Vol.5, 1971
- Vol.4, 1970-1971
- Vol.3, 1969-1970
- Vol.2, 1968
- Vol.1, 1966-1967




